Chemistry of
Catechol Sulfates. Regioselective Ring Opening and Stabilization of Reaction
Product by Hydrogen Bonding.
Zoltan G. Hajos a
Formerly at
Abstract
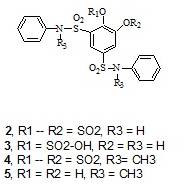
The chemistry of catechol sulfonanilides 2 and 4 has been reinvestigated. It was found that the cyclic sulfate
ring of the sulfonanilide 2 and the
C-1 sulfonanilide group would regioselectively hydrolyze in refluxing aqueous
acetone with aniline to the catechol O-sulfonic acid 3 stabilized by intramolecular H-bonding. On the other hand, the cyclic sulfate of the N-methyl
sulfonanilide 4 remains unchanged in
refluxing aqueous acetone with N-methyl
aniline. It undergoes, however, hydrolysis to intermediate 4A, the N-methyl analog of compound 3 by refluxing it in aqueous acetone in the presence of aniline.
Intermediate 4A is not stabilized by
intramolecular H-bonding; it undergoes therefore hydrolysis to the catechol
derivative 5.
Keywords: benzenesulfonanilides,
catechol sulfate derivatives, regioselective hydrolysis, intramolecular
H-bonding, inhibition of hydrolysis in aqueous media.
Introduction
A paper by Pollak and Gebauer-Fülnegg concerning
the chemistry of cyclic catechol sulfate derivatives appeared in 1926.
Twentyseven years later, because of the interest in catechol and homo
pyrocatechol derivatives at the
Results and Discussion
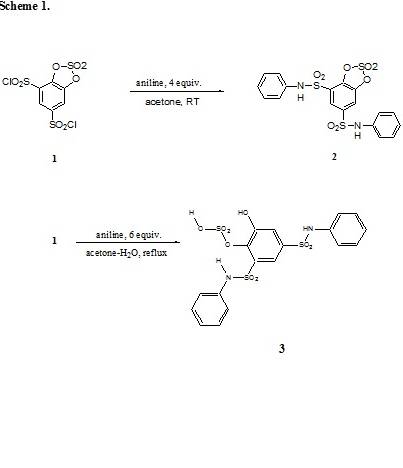
The authors of the 1926 paper reacted
compound 1, the disulfonyl chloride
of catechol cyclic sulfate with an excess of aniline in refluxing acetone. They
filtered the reaction mixture and crystallized the precipitate by refluxing it
in aqueous acetone. Upon cooling they claimed to have obtained compound 2, mp at about 304o C with
decomposition. They characterized the reaction product by nitrogen
determination only (N, 5.67-6.0) 1. By repeating the original
procedure, we found the correct structure to be the water insoluble compound 3. Its melting point was identical to
that reported earlier 1 (mp 304o C, dec.). Compound 2 and compound 3 have rather similar N-values. Nitrogen determination alone is
therefore insufficient to characterize these compounds.
The cyclic catechol sulfate group of
compound 2 hydrolyzed by
regioselective ring opening due to the anchimeric assistance of the ortho
standing sulfonanilide group. This was responsible for the stereoselectivity of
the hydrolytic attack of the aniline/ aniline-hydrochloride system in the
refluxing aqueous acetone medium. The resulting catechol O-sulfonic acid 3 was stabilized by intramolecular
H-bonding. Also, since the compound was practically insoluble in water its
precipitation protected it from further hydrolysis. O-sulfonic acid derivatives
of catechol have been described in the scientific literature.4a,b The reaction therefore constitutes an example of inhibition
from hydrolysis in the presence of water due to insolubility of the substrate
in water and its stabilization by intramolecular hydrogen bonding. The results
are shown in Scheme 1.
In modern organocatalysis water insoluble
proline derivatives have been successfully used to catalyze intermolecular
aldol reactions in water.5
The facile
opening of the five-membered ring catechol cyclic sulfate has been well
documented in the scientific literature.6 A paper entitled: “Intramolecular Nucleophilic
Catalysis by the Neighboring Hydroxyl Group in Acid-Catalyzed
Benzenesulfonamide Hydrolysis”7 describes
the regioselective hydrolysis of a sulfoanilide group assisted by a vicinal
hydroxyl group.
Using the theoretically
necessary four equivalents of aniline, and by executing the reaction at RT in
dry acetone we obtained the cyclic catechol sulfate 2. It analyzed correctly for C, H, N and S, and had mp of 125°C. Its 1H
NMR spectrum was in good agreement with structure 2. The compound was thus clearly different from the one described
earlier1.
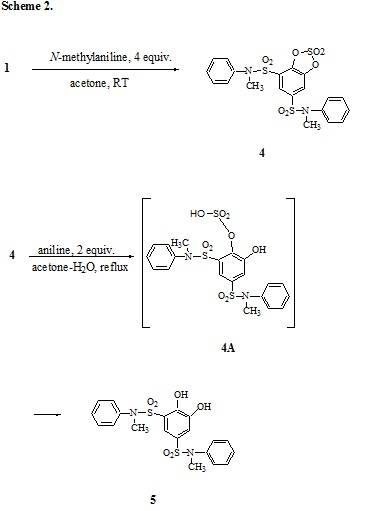
Pollak and
Gebauer-Fülnegg also described treatment of 1 with N-methylaniline to
give the cyclic catechol sulfate ester 4,
the N-methyl analog of 2. Compound 4 had mp 146°C, and was again characterized by
nitrogen determination only 1. Using our procedure (4 equivalents of
N-methylaniline in acetone at RT), we
too obtained compound 4, mp 145-146°C, and
confirmed its structure by C, H, N and S analysis and 1H NMR. While
nitrogen determination can practically not differentiate between compounds 2 and 3, the C, H and S values of these two compounds are clearly
different.
The cyclic
sulfate of the N-methyl sulfonanilide 4
remains unchanged in refluxing aqueous acetone with N-methyl aniline 1, but undergoes hydrolysis to the
intermediate 4A, the N-methyl analog
of 3
in the presence of aniline under identical reaction conditions. Since 4A is not being stabilized by H-bonding
it undergoes hydrolysis to the catechol 5,
mp 86°C. It analyzed correctly for C, H, N and S. Its 1H
NMR spectrum was in good agreement with structure 5. Compound 5 is new.
The conversion of the cyclic catechol sulfate 4 to the catechol 5 most
likely proceeds via the 4A-type
sulfonic acid derivative, the N-methyl analogue of compound 3. However, unlike compound 3 intermediate 4A is not being stabilized by intramolecular H-bonding, and is
therefore readily converted to compound 5.
It is interesting to note that the cyclic catechol sulfate ring does not open
with N-methyl aniline, but opens readily with aniline a primary amine. The
results are shown in Scheme 2.
Experimental Section
General
Procedures.
Melting point determinations were done on
a Thomas Hoover capillary melting point apparatus and are uncorrected.
Nuclear-magnetic-resonance (1H NMR) spectra were recorded on a Varian
HR 100 spectrometer. Chemical shifts (d) are reported in parts per million downfield relative to
tetramethylsilane as a standard. All new compounds gave consistent 1H NMR spectra.
1,3,2-benzodioxathiole-2,2-dioxide-4,6-disulfonyl
dichloride (1). Compound
1 has been prepared by the
literature procedure1 in 47.7% yield. Mp 141-143 oC (lit.
1 mp 143 oC).
1H NMR (100 MHz, DMSO-d6) d 7.6 (1H, d), 8.0 (1H, d). Anal. Calcd.
for C6H2Cl2O8S3:
C,19.52; H, 0.55; S, 26.06. Found: C, 19.30; H, 0.62; S, 25.91.
1,3,2,benzodioxathiole-2,2-dioxide-4,6-disulfonic
acid bis-phenylamide (2).
To compound 1 (1.8 g, 4.9 mmol)
dissolved in acetone (5 mL) was added dropwise aniline (1.8 g, 19.6 mmol)
dissolved in acetone (5 mL). It was allowed to stand at RT for 16 h., filtered,
and the solvent was evaporated in vacuo. The residue was dissolved in
chloroform, and washed with water to remove aniline hydrochloride. It was then
washed with brine, dried over sodium sulfate, and concentrated
in vacuo to give an oily solid (2.4 g, 4.9 mmol). Recrystallization from
ether-hexane gave compound 2 (1.8 g,
76%) needles, mp 123-125 oC; 1H NMR (100 MHz, CDCl3)
d 4.0 (2NH, s), 6.5 (4H,
d), 6.6 (2H, d), 7.0 (4H, d), 7.2 (1H, d), 8.0 (1H, d). Anal. Calcd. for C18H14N2O8S3:
C,44.81; H, 2.92; N, 5.81; S, 19.94. Found: C, 44.75; H, 3.00; N, 5.80; S,
19.86.
Sulfuric
acid mono-(2-hydroxy-4,6-bis-phenylsulfamoyl-phenyl) ester (3).
To compound 1 (3.0 g, 8.1 mmol) in acetone (5 mL)
was added dropwise aniline (4.5 g, 48.4 mmol) dissolved in acetone (10 mL). The
reaction turned slightly warm, and a thick precipitate formed. It was then
filtered, and washed with a small amount of acetone to remove the excess
aniline. The solid was dispersed in aqueous acetone (20 mL), and it was boiled
for 30 min. It was cooled to RT, and filtered. The water insoluble solid has
been compound 3 (3.0 g, 75%), mp 304o
C, dec. 1H NMR (100 MHz, DMSO-d6)
d 2.0 (1H, s), 4.0 (2H,
s), 5.0 (1H, s), 6.46 (4H, m), 6.6 (2H, d), 7.0 (4H, d), 7.2 (1H, d), 8.0 (1H,
d). Anal. Calcd. for C18H16N2O9S3:
C,43.19; H, 3.22; N, 5.60; O, 28,77; S, 19.22. Found: C, 43.20; H, 3.20; N,
5.66; S, 19.20.
1,3,2-Benzodioxathiole-2,2-dioxide-4,6-disulfonic acid
bis-(methyl-phenyl-amide) (4). To
compound 1 (1.8 g, 4.9 mmol)
dissolved in acetone (5 mL) was added dropwise N-methylaniline (2.1 g, 19.6 mmol) dissolved in acetone (5 mL). It
was allowed to stand at RT for 16 h. Work up similar to that of compound 2 gave compound 4 (2.1 g, 85%) needles, mp 145-146 oC (lit1
mp 146 oC); 1H NMR (100 MHz, CDCl3) d 2.8 (2NCH3, d), 6.4 (4H, d),
6.6 (2H, d), 7.0 (4H, d), 7.2 (1H, d), 8.0 (1H, d). Anal. Calcd. for C20H18N2O8S3:
C, 47.05; H, 3.55; N, 5.49, S, 18.84.
Found: C, 47.00; H, 3.50; N, 5.50 (lit2 N, 5.63), S, 18.80.
4,5-Dihydroxy-benzene-1,3-disulfonic
acid bis-(methyl-phenyl-amide),
5. To compound 4 (1.0 g, 1.96 mmol) dissolved in acetone (5.0 mL) was added
aniline (3.6 g, 3.9 mmol) dissolved in acetone (5.0 mL). To the resulting
solution was added water (5.0 mL), and it was refluxed for 1h. The solvent was
evaporated in vacuo. The residue was dissolved in ether, and washed with water
to remove the aniline sulfate byproduct. It was then washed with brine, dried
over sodium sulfate, and concentrated in vacuo to give a solid (0.8 g, 1.8
mmol). Recrystallization from ether-hexane gave compound 5 (0.7 g, 79%) needles, mp 86oC; 1H NMR (100
MHz, CDCl3 ) d 2.8 (2NCH3,
d), 5.0 (2OH, s), 6.4 (4H, d), 6.6 (2H, d), 7.0 (4H, d), 7.2 (1H, d), 8.0 (1H,
d). Anal. Calcd. for C20H20N2O6S2:
C, 53.56; H, 4.49; N, 6.25, S, 14.30.
Found: C, 53.40; H, 4.50; N, 6.20; S, 14.25.
References
a.
Private
communication by Zoltan G, Hajos,
1.
Pollak, J.; Gebauer-Fülnegg, E., Monatshefte, 1926, 47, 109
2.
Kendall, E.C.; Hajos, Z.G., J.Am.Chem.Soc., 1960, 82, 3219.
3. Zheng, X.; Oda; H.; Harada, S.; Sugimoto,
Y.; Tai, A.; Sasaki, K.; Kakuta, H., J. Pharm. Sci., 2008, 97, 5446-5452.
4. a. Cerfontain, H.;
5. Qingquan Zhao, Yu-hong Lam, Mahboubeh Kheirabadi, Chongsong Xu, K.N. Houk and
Christian E. Schafmeister, Hydrophobic Substituent Effects on Proline Catalysis
of Aldol Reactions in Water, J. Org. Chem., 2012, 77 (10), pp 4784–4792 DOI: 10.1021/jo300569c
6. Emil Thomas Kaiser, Acccounts Chem.Res., 1970, 3, 145.
7. Wagenaar, Anno; Kirby, Anthony J.;
Engberts, Jan B.F.N. J.Org.Chem., 1984,
49, 3445.
Graphical Abstract
|
Chemistry of Catechol Sulfate Derivatives Zoltan G. Hajos |
|
To see the Scientific Activities of Zoltan G. Hajos click on:
http://www.dobroka.hu/zghajos/index.htm
.